Experience the interactive Multimedia News Release here http://www.multivu.com/players/English/7223451-boston-scientific-spyglass-ds/
More than one million people worldwide undergo endoscopic retrograde cholangiopancreatography (ERCP) procedures each year to diagnose and treat diseases and conditions of the liver, gallbladder, pancreas and bile ducts.1 In the event that x-ray imaging is not sufficient to make a definitive diagnosis or therapeutic intervention requires direct visualization, cholangioscopy or pancreatoscopy may be performed. Cholangioscopy is the examination of the bile ducts using an endoscope to enable direct visualization of the biliary tree during ERCP while pancreatoscopy is the examination of the pancreatic ducts. Direct visualization of the bile and pancreatic ducts during ERCP can help obtain biopsy specimens, lead to the diagnosis of abnormalities, and guide stone therapy.2
"I'm extremely pleased with the overall functionality of the new SpyGlass DS System," said Robert Hawes, M.D., FASGE, The Center for Interventional Endoscopy at Florida Hospital Orlando. "It was quick and easy to set up ('plug and play'), the image quality and stability excellent and the four-way tip deflection intuitive. The system now enables endoscopists with ERCP expertise to perform cholangioscopy with or without intervention. My expectation is that this technology will increase our ability to diagnose and treat pancreatobiliary diseases and reduce the number of repeat ERCPs."
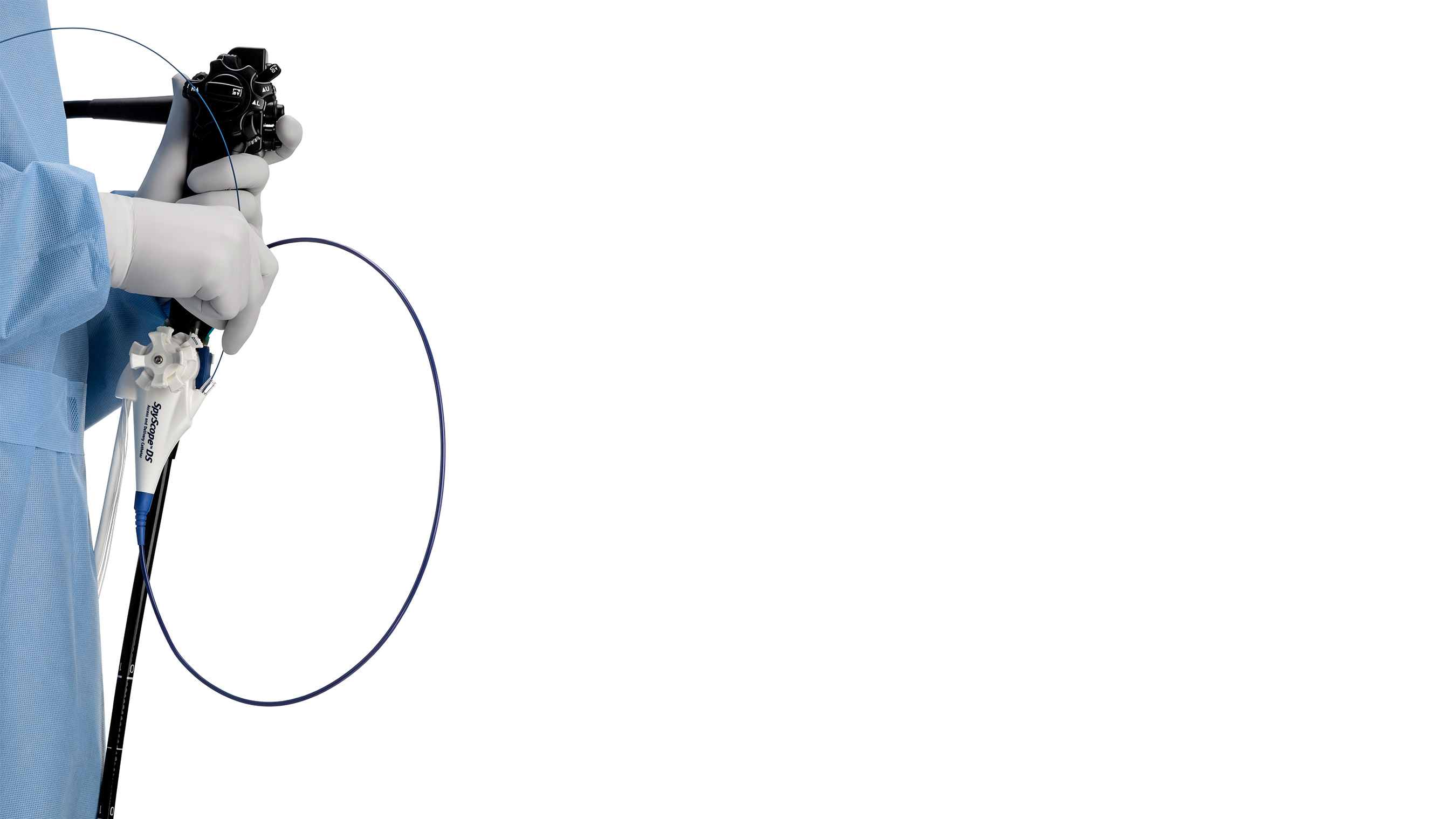
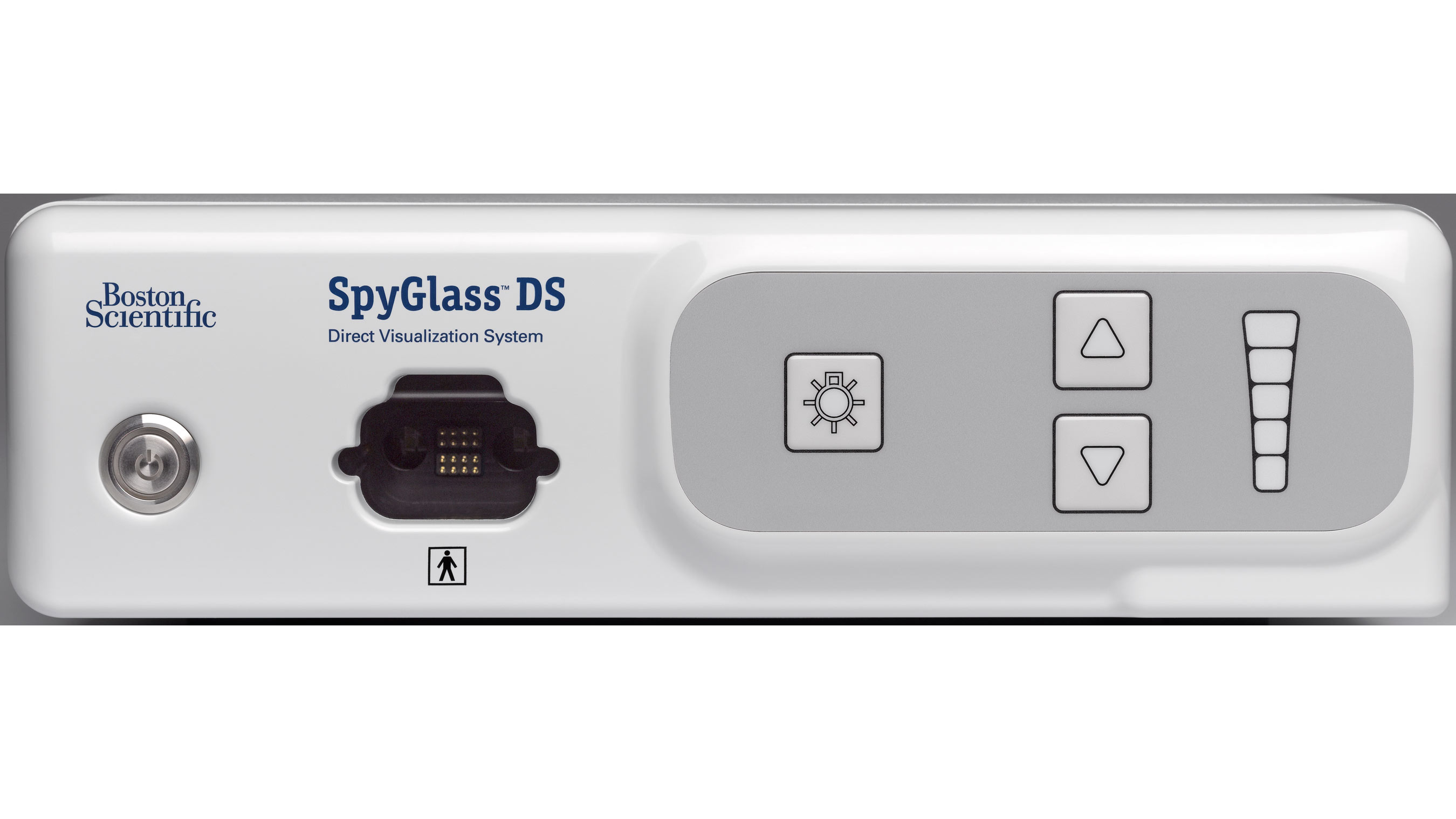
Launched in 2007, the original SpyGlass System helped re-establish cholangioscopy and pancreatoscopy as a valuable diagnostic and therapeutic procedure by allowing a single physician to perform the procedure as well as guide devices to examine, diagnose and treat conditions such as gallstones and suspected malignancies of the biliary tree and pancreas. The new SpyGlass DS System builds on this technology with enhanced features to further improve visualization and help simplify the procedure. The system consists of a fully integrated SpyScope™ DS Access and Delivery Catheter, and a single-use scope to eliminate probe reprocessing and image degradation over multiple uses. The integrated digital sensor provides superior imaging, far greater resolution and a 60 percent wider field of view than the first generation system. In addition, the SpyGlass DS System offers physicians an integrated controller that fits on a standard ERCP cart for improved accessibility and 'plug and play' setup, helping to reduce procedure time.
"Early detection is critical to improving outcomes in patients suffering from difficult pancreatico-biliary diseases, such as pancreatic cancer," said David Pierce, senior vice president and president, Endoscopy, Boston Scientific. "The new SpyGlass DS System can be performed as an extension of any ERCP procedure, enabling physicians to diagnose and treat more of their patients effectively and efficiently. Boston Scientific is proud to bring single-operator cholangioscopy to a new level of treatment."
1 Chen Y et al, Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc 2011; 74:805-814.
2 CPT Copyright 2014 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding new product launches and launch cadence, regulatory approvals, markets for our products, product performance and impact and competitive offerings. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Media: Nisha Deo
408-893-9243 (cell)
Global Media Relations
Boston Scientific Corporation
Email Contact
Investors: Susie Lisa, CFA
508-683-5565 (office)
Investor Relations
Boston Scientific Corporation
Email Contact

To view the original version on PR Newswire, visit: http://www.prnewswire.com/news-releases/boston-scientific-launches-next-generation-spyglass-ds-direct-visualization-system-for-advanced-diagnosis-and-treatment-of-pancreatico-biliary-diseases-300041604.html
SOURCE Boston Scientific Corporation