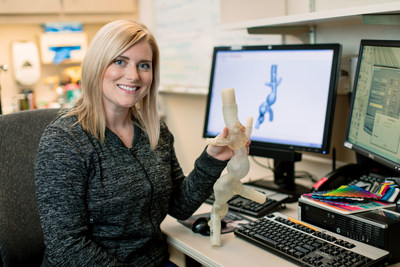
As one of fastest-growing AM/3DP application areas, even more growth is expected for biomedical/medical applications. More than 97 percent of medical AM/3DP professionals expect the use and application to continue to grow (SME Medical AM/3DP Survey, December 2017). Once again, RAPID + TCT's Medical Manufacturing Innovations Program (MMI) will highlight this growth through conference sessions and other programming. The event has also become a yearly meeting place for workgroups such as the SME Medical Additive Manufacturing/3D Printing Workgroup, the Radiological Society of North America (RSNA) 3D Printing Special Interest Group, and the Digital Imaging and Communications in Medicine (DICOM) Workgroup 17.
"RAPID + TCT's collaborative approach gives stakeholder groups an opportunity to identify challenges, develop resources, and to facilitate changes to support anyone using the technologies for medical/biomedical applications," said Lauralyn McDaniel, Medical Additive Manufacturing Industry Expert, SME. "Applications are getting more complex; we're focusing on issues ranging from working within a clinical setting for point-of-care manufacturers and regulatory/quality requirements to the developing area of tissue fabrication and bioprinting."
Mayo Clinic: Impacting More Patients with 3D Printing
From the clinical side, Mayo Clinic has become a leader in point-of-care manufacturing, establishing their first 3D printing lab in 2006. Using medical imaging data, the Mayo Clinic Radiology department has worked with surgeons and other healthcare professionals to manufacture anatomical models, surgical guides, and more. 3D POC manufacturing has risen in the last few years supported by advancements in machines, materials, software, and awareness. Join Jonathan M. Morris, MD, Mayo Clinic, and Amy Alexander, BME, Mayo Clinic on Wednesday morning to learn how point-of-care manufacturing is providing patient care advantages, presenting unique obstacles of engineering within a hospital, creating the need for collaboration across disciplines, and impacting the lives of many patients and their families.
Regulating 3D Printed Medical Devices
Along with keynotes and panel discussions, RAPID + TCT's MMI program offers three focused workshops: "Regulatory and Quality System Considerations for 3D Printed Medical Devices" addressing the needs of medical device manufacturers; "3D Printing in Hospital" focusing on the needs of POC manufacturers; and "Biomaterials and Bioprinting Fundamentals & Applications," looking to the next generation of 3D-printed products to improve patient care. As the number of medical applications continues to grow, these workshops bring together technology and process experts to walk participants through the considerations at every step.
To learn more about the event and presentations, please visit http://www.rapid3devent.com/.
About RAPID + TCT
For over 25 years, RAPID has defined the crucial role of additive manufacturing and empowered the establishment of an industry that continues to conceive, test, improve and manufacture new products at a faster, more cost-efficient pace. For users and suppliers alike, the event will be the premier destination for those who provide technology and for those who need to understand, explore and adopt 3D printing, additive manufacturing, 3D scanning, CAD/CAE, metrology and inspection technologies. For more information, please visit
rapid3devent.com.
About SME Medical Additive Manufacturing/3D Printing Workgroup
Making a difference through collaboration
The SME Medical Additive Manufacturing/3D Printing Workgroup supports users of medical and biomedical application technology. Members represent medical device manufacturers, clinicians, technology providers and more, including Mayo Clinic, Biomet, University of Michigan, Smith & Nephew, Materialise, nScrypt, Leuven Medical Technology Centre, DePuy Synthes, Stryker Orthopaedics, Phoenix Children's Hospital, Johnson & Johnson and Northwestern University. By providing content to address the latest industry developments, identify gaps in standards, and build evidence for additive manufacturing applications in medicine, the group helps drive technology to improve and save lives. For more information, please visit
sme.org/medical-am3dp-workgroup.
About SME
SME connects manufacturing professionals, academia and communities, sharing knowledge and resources to build inspired, educated and prosperous manufacturers and enterprises. With more than 85 years of experience and expertise in events, media, membership, training and development, and also through an education foundation, SME is committed to promoting manufacturing technology, developing a skilled workforce and attracting future generations to advance manufacturing. Learn more at
sme.org, follow
@SME_MFG on Twitter or
facebook.com/SMEmfg.

![]() View original content with multimedia:
http://www.prnewswire.com/news-releases/rapid--tct-2018-collaborating-to-advance-medical-additive-manufacturing-300606813.html
View original content with multimedia:
http://www.prnewswire.com/news-releases/rapid--tct-2018-collaborating-to-advance-medical-additive-manufacturing-300606813.html
SOURCE SME
| Contact: |
| Company Name: SME
Ashley Areeda, Senior PR Representative, SME, 734-891-4013 Email Contact Web: http://www.sme.org |